Neurotechnology development
(In questions of science, the authority of a thousand is not worth the humble reasoning of a single individual.*)
Adaptive optics to image boutons and spines in deep layers of neocortex and somata in the brainstem
We advanced two-photon microscopy for near diffraction-limited imaging along the full depth of cortex in awake behaving mice and somata throughout the highly scattering medulla in anesthetized preparations. Our approach combines direct wavefront sensing of descanned fluorescence from dye introduced in brain microvessels, which forms a guide-star, and adaptive optics (AO) to compensate for tissue-induced aberrations of the wavefront.
In neocortex, we achieve high signal-to-noise records of glutamate release from thalamocortical boutonss, using expression of iGluSnFR3, and high signal-to-noise records of intracellular [Ca2+] transients in individual spines of layer 5b basal dendrites, using expression of jRGECO1a.
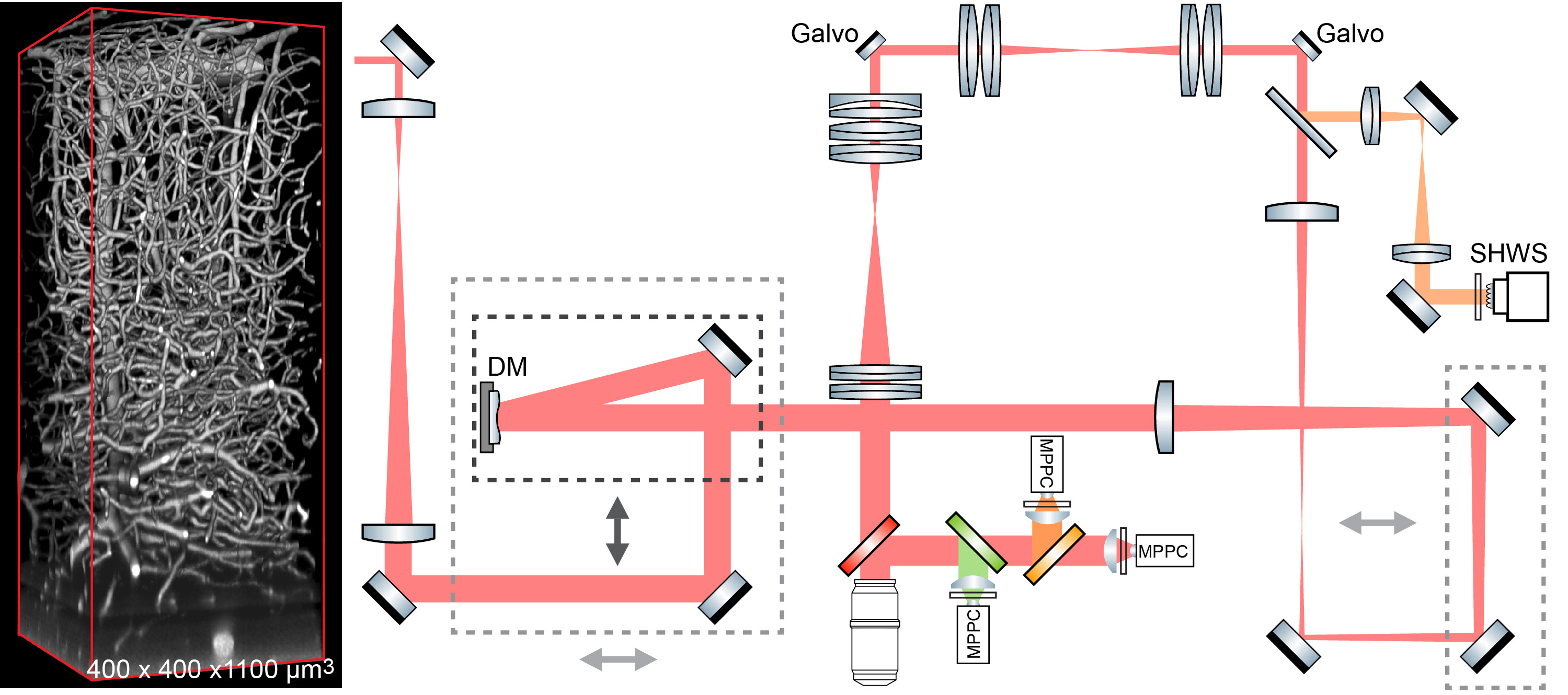
Extensions of this work involve the incorporation of fast shifts in focal plane with AO corrections and power modulation at each plane, pulse splitting for elongated beams, and concurrent opto-genetics and/or opto-ablation.